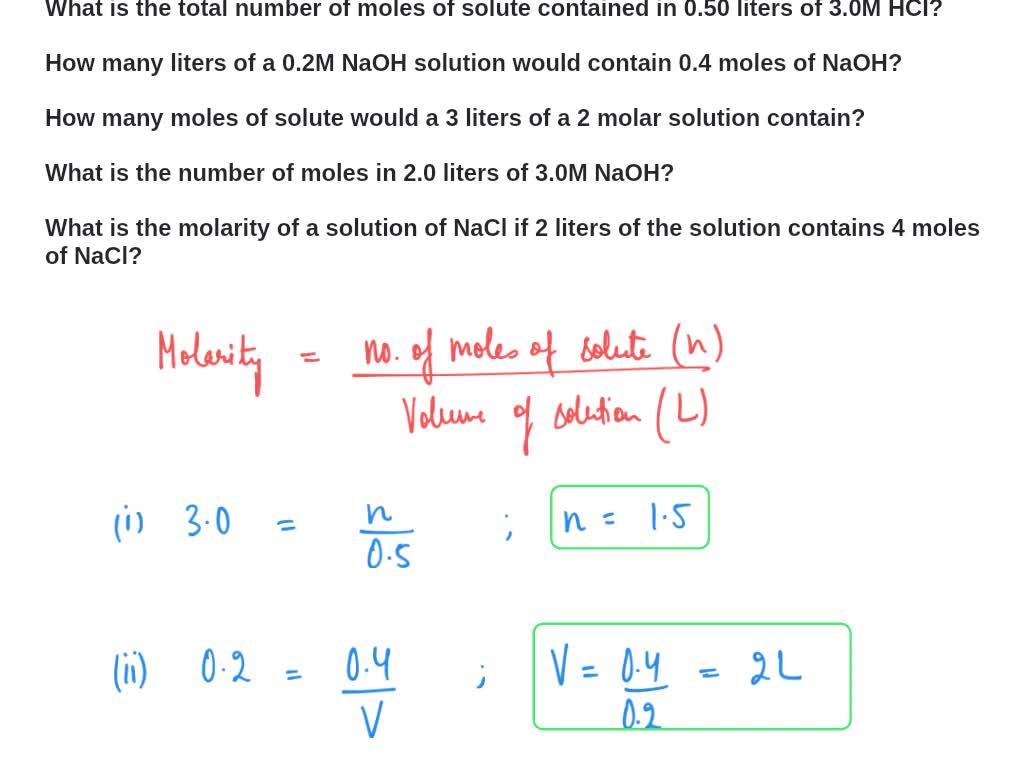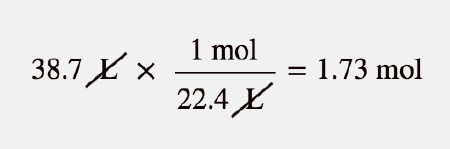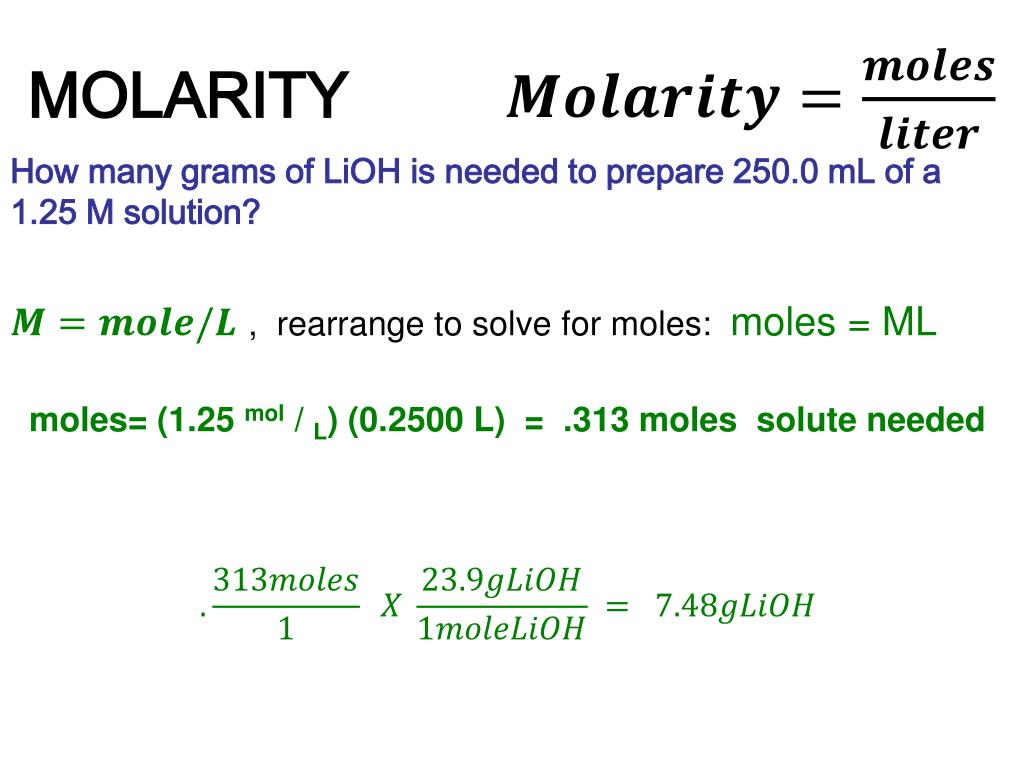
PPT - MOLARITY A measurement of the concentration of a solution Molarity (M) is equal to the moles of solute (n) per liter of PowerPoint Presentation - ID:1459925

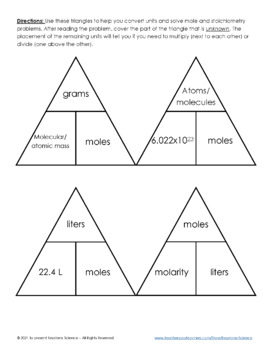
Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry - Y… | Stoichiometry chemistry, Chemistry worksheets, Chemistry

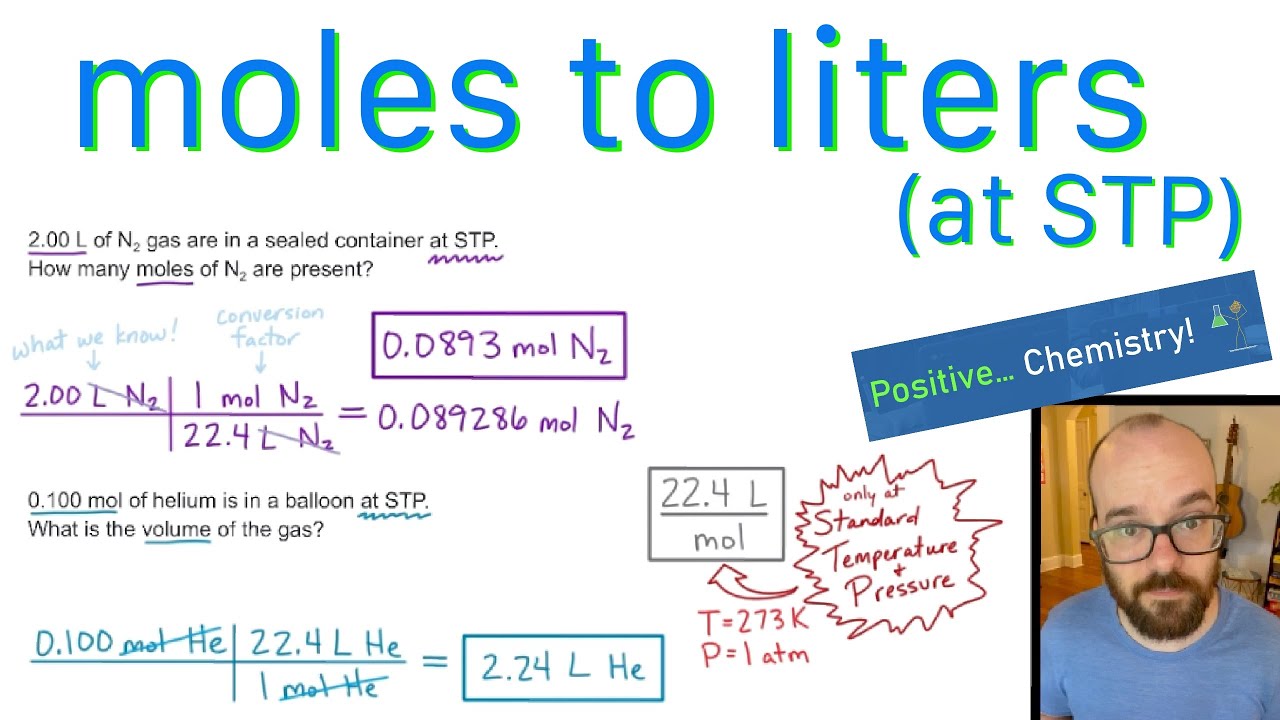
SOLVED:Use molar volume to calculate each of the following at STP: a. the volume, in liters, occupied by 2.50 moles of N2 gas b. the number of moles of CO2 in 4.00
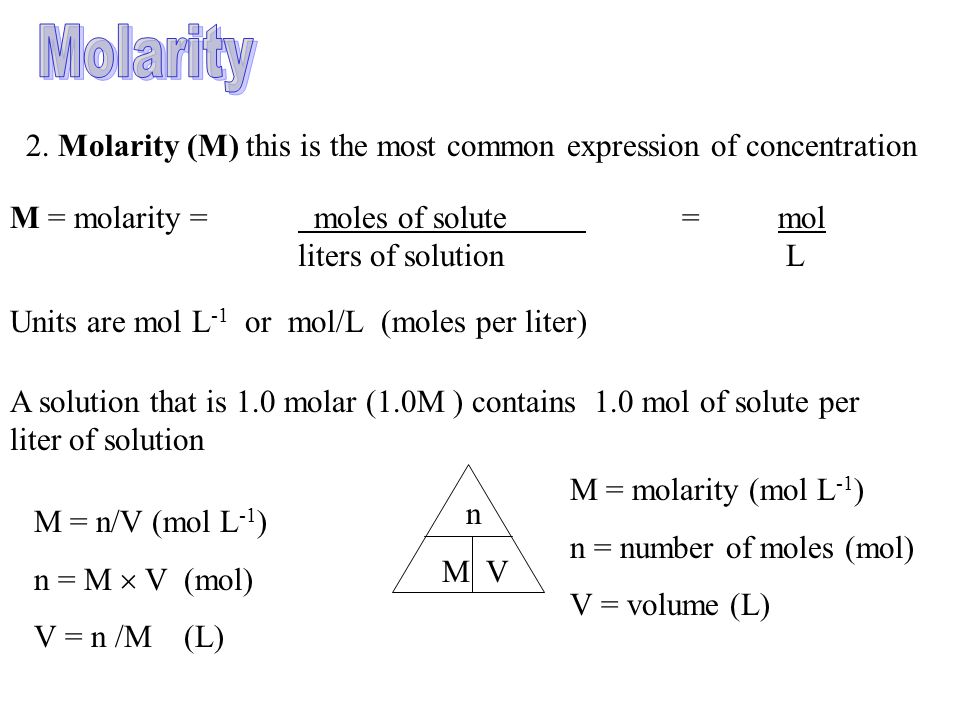
Molarity 2. Molarity (M) this is the most common expression of concentration M = molarity = moles of solute = mol liters of solution L Units are. - ppt download

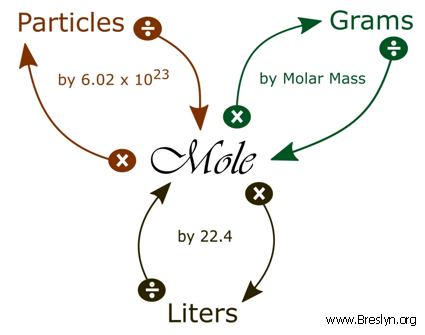
Answer Key Moles & Liters volume .docx - Moles & Liters Volume We've established that it's not practical to count atoms so we often need to use | Course Hero