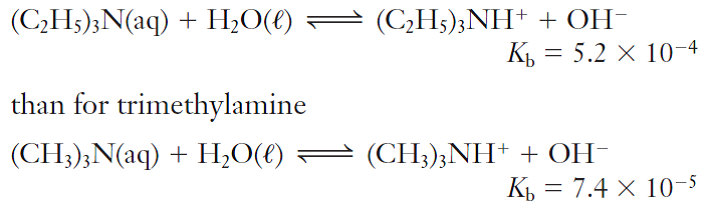18856-86-5 | Trimethylamine-d9 Hydrochloride | N,N-Dimethylmethanamine-d9 Hydrochloride; | C₃D₉N • HCl | TRC
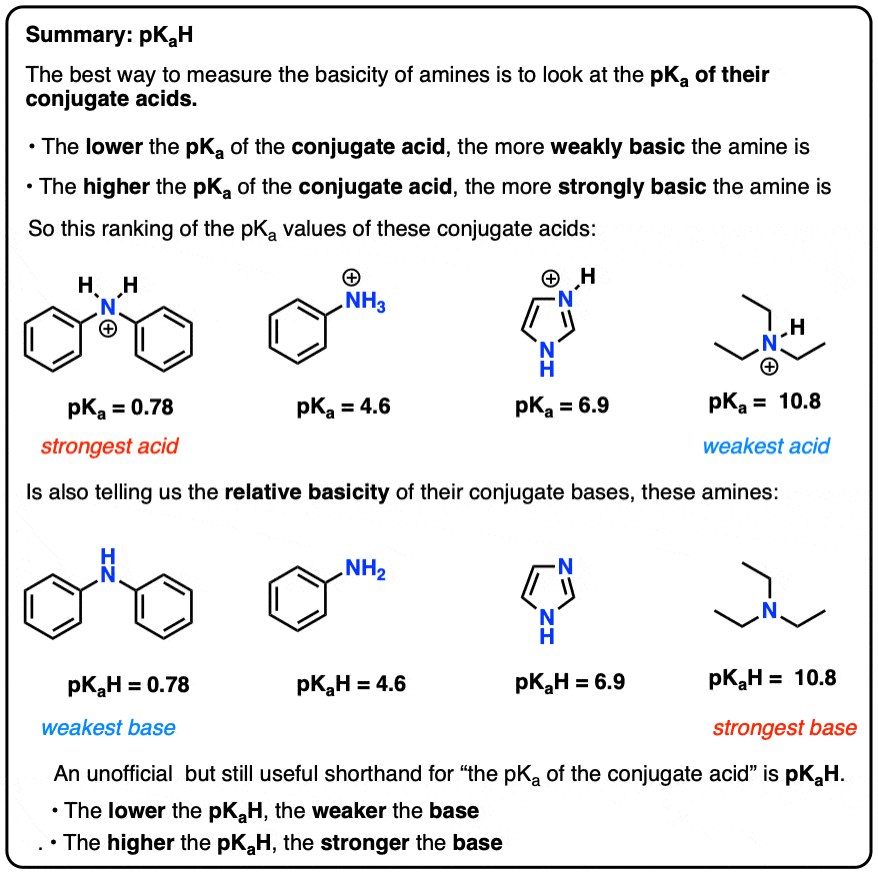
Which among the following is strongest base in gas phase? (a) Triethylamine (b) Diethylamine (c) Ethylamine (d) Ammonia

Calculate the pH of a 0.443 M aqueous solution of trimethylamine. (Kb = 6.3 x 10-5) | Homework.Study.com

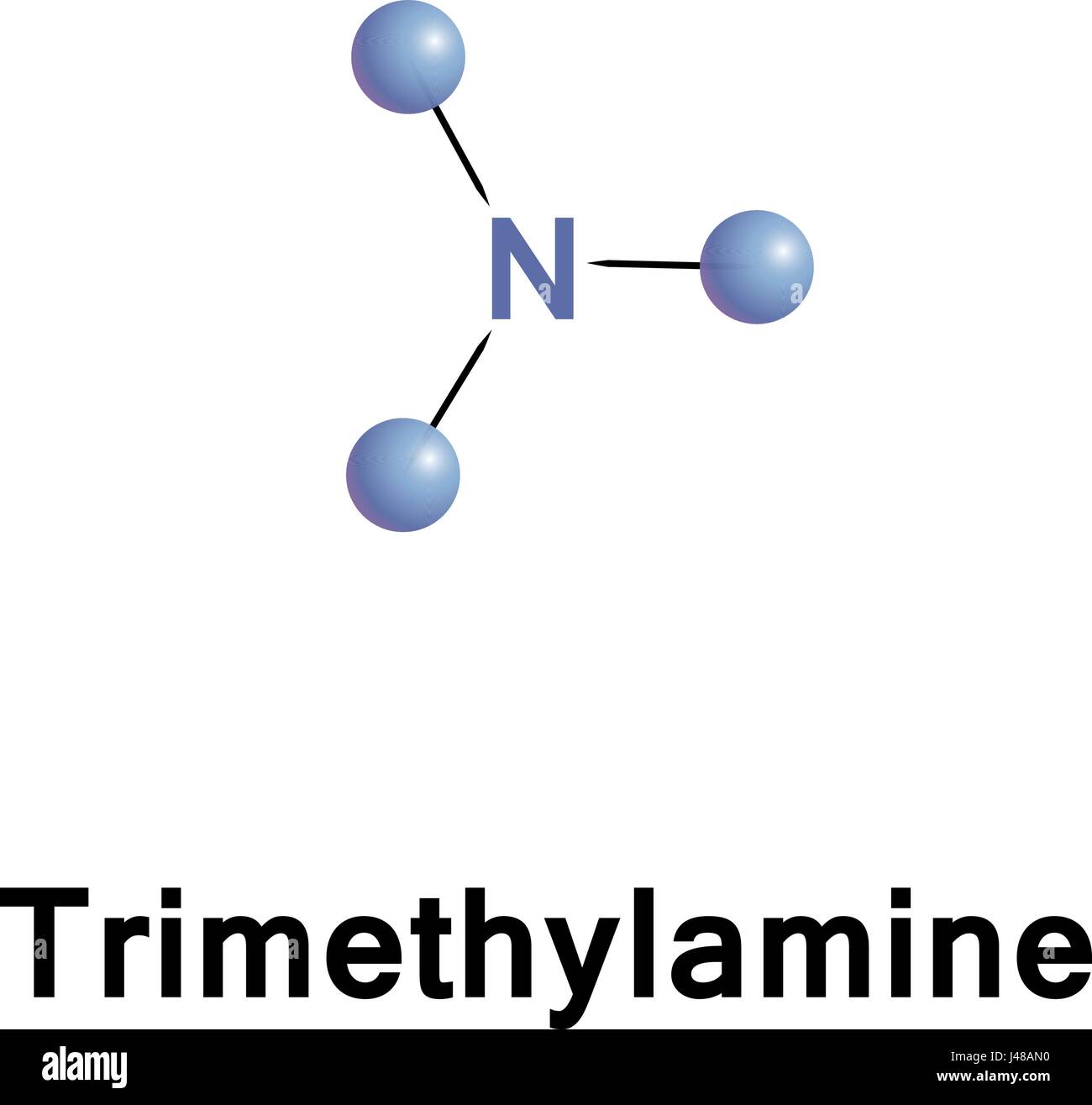
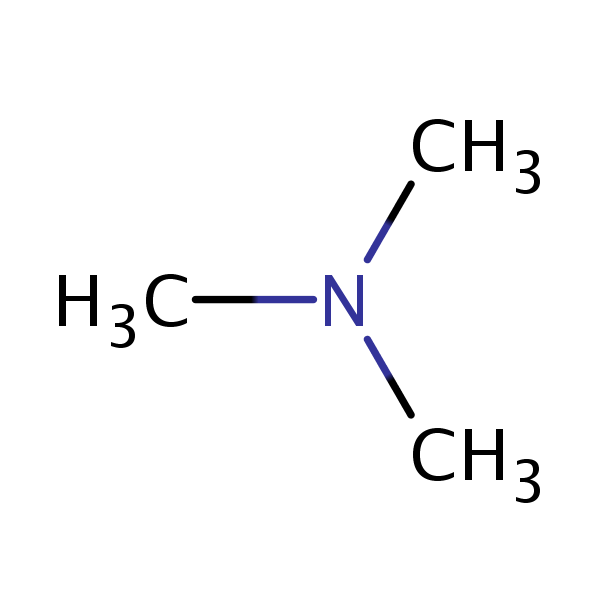
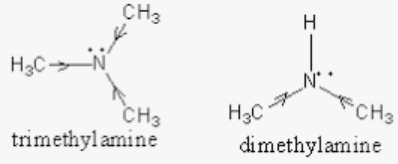
mass spectrum of N,N-dimethylmethanamine (trimethylamine) C3H9N (CH3)3N fragmentation pattern of m/z m/e

Trimethylamine Is A Nitrogenous Base And Can Be Protonated To Trimethylammonium Cation. This Colorless, Hygroscopic, And Flammable Tertiary Amine Has A Strong Fishy Or An Ammonia-like Odor Stock Photo, Picture And Royalty

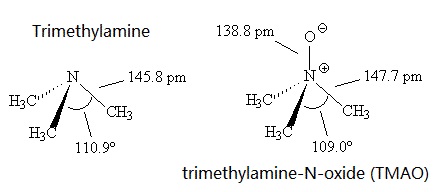
Welcome to Chem Zipper.com......: Why trimethylamine amine ( N(CH3)3) is tetrahedral while trisilyl amine (N(SiH3)3) planner.?
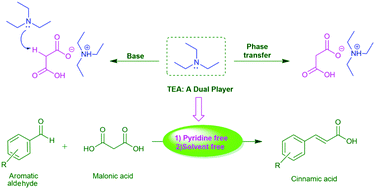
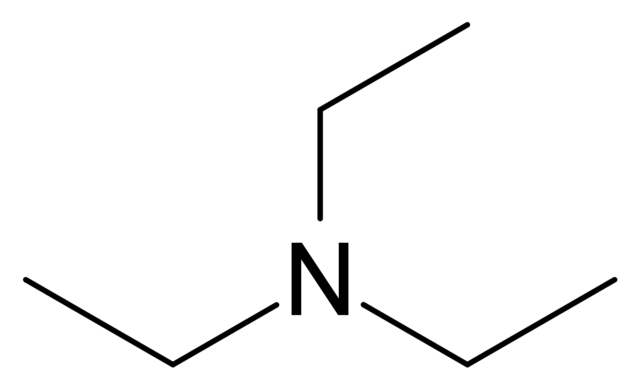
Triethylamine: a potential N-base surrogate for pyridine in Knoevenagel condensation of aromatic aldehydes and malonic acid - New Journal of Chemistry (RSC Publishing)

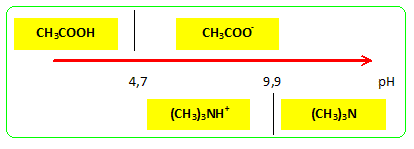
Trimethylamine, (CH3)3N, is a weak base that ionizes in aqueous solution: (CH3)3N(aq) + H2O(l) - Brainly.com
✓ Solved: Base Ionization Trimethylamine, (CH3)3N, is a gas with a fishy, ammonialike odor. An aqueous...