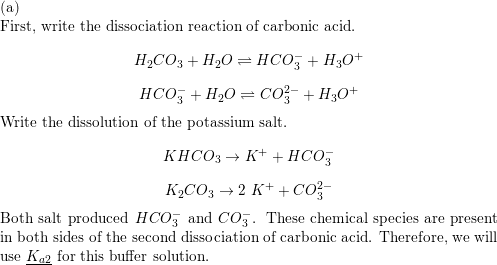Difference Between Potassium Carbonate and Potassium Bicarbonate | Compare the Difference Between Similar Terms
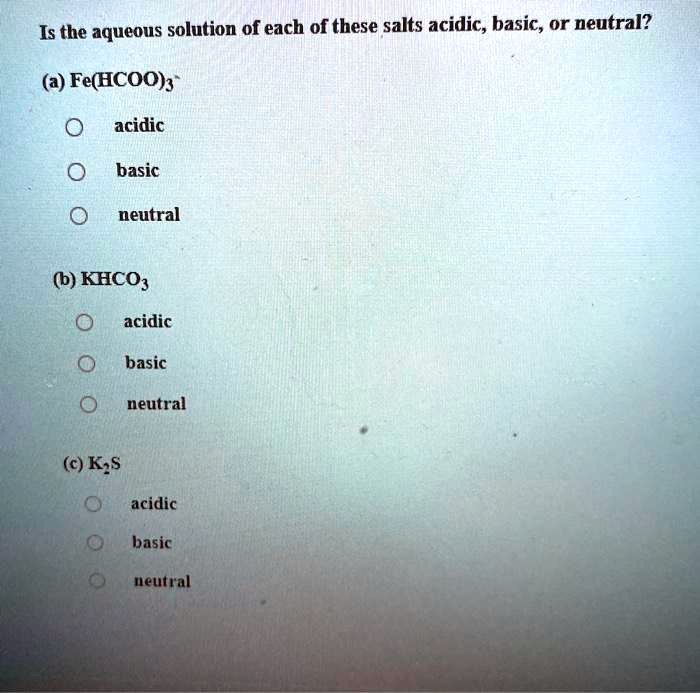
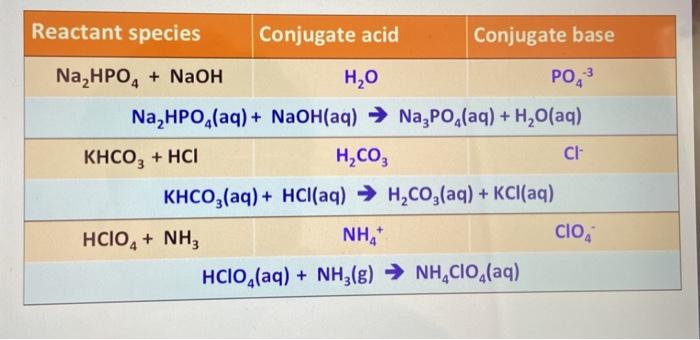
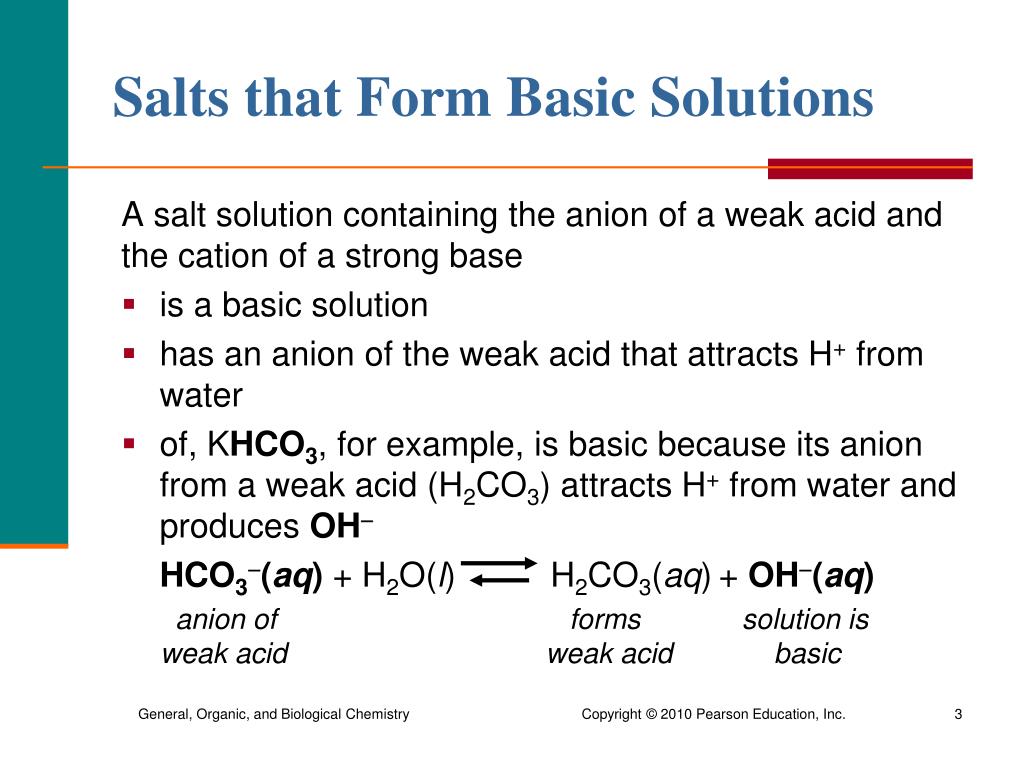
SOLVED: Is the aqueous solution of each of these salts acidic, basic, or neutral? (a) Fe(HCOO)s acidic basic neutral (b) KHCO acidic basic neutral (c) K;s acidic basic ueutral

Iodine Mediated Base‐Controlled Regio‐Selective Annulation of 2‐(Pyridin‐2‐yl)acetate Derivatives with Acrylic Esters for the Synthesis of Indolizines - Fang - 2020 - Advanced Synthesis & Catalysis - Wiley Online Library