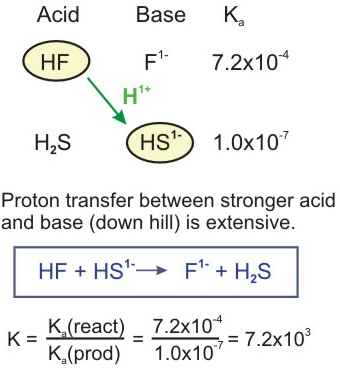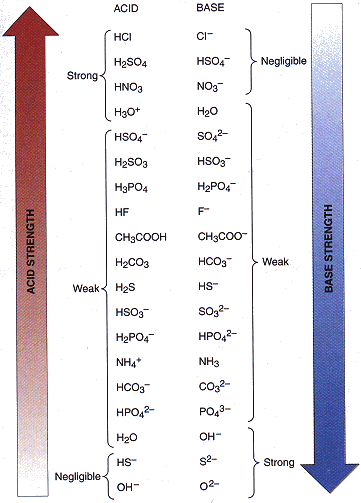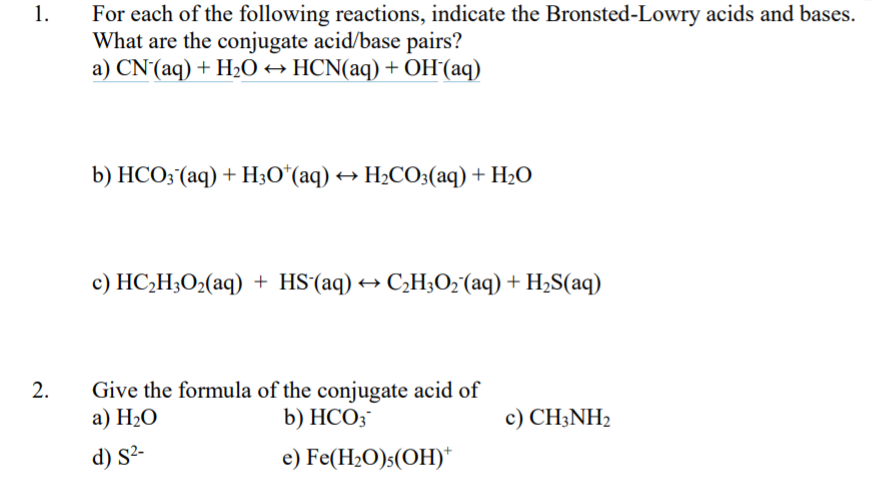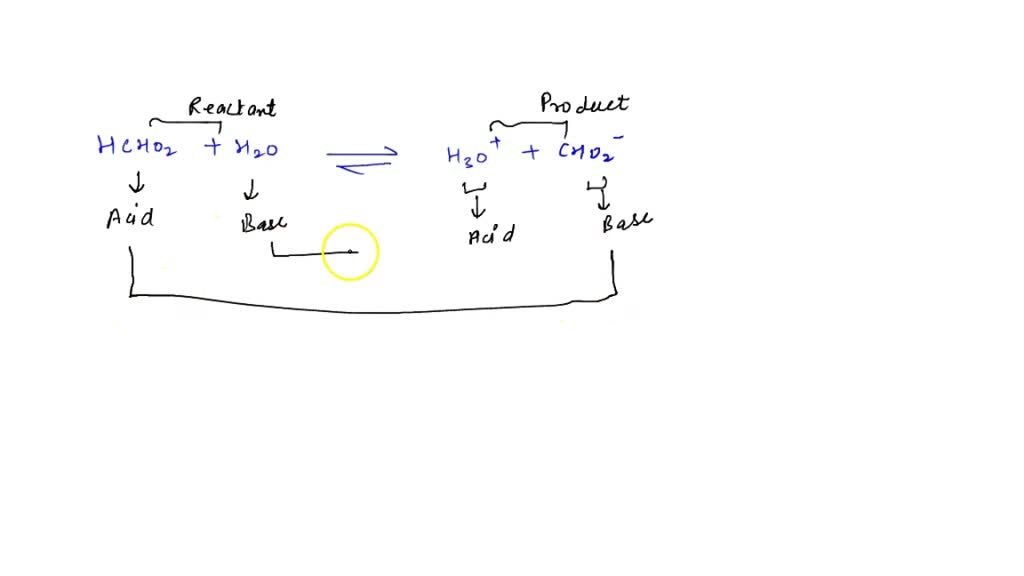
SOLVED: In aqueous solution, HS- is the conjugate acid of and the conjugate base of . Group of answer choices H2S, S2- H3O+, S2- S2-, H2S S2-, H3O+

Experts please tell whether H2S is lewis acid or lewis base and bronsted acid or bronsted base - Chemistry - Hydrogen - 16684677 | Meritnation.com

SOLVED: Write an equation that shows the reaction of hydrogen sulfide, HS– with hydroxide ion, OH–. Label the acid, the base, the conjugate acid, and the conjugate base.

Which of the species from the equilibrium below are conjugate acid-base pairs? NH3(aq) + H2S(aq) arrow HS-(aq) + NH4+(aq) | Homework.Study.com

SOLVED: Identify the conjugate acid base pairs in each of the following: 1. HS-(aq) + H2O(l) ⇌ H2S(aq) + OH-(aq) 2. H2S(aq) + NH3(aq) ⇌ NH4(aq) + HS-(aq) 3. H2SO4(aq) + H2O(l)